Bohr's atomic model
Neil Bohr's Atomic Model is an improvement of Rutherford's Atomic Model and was introduced by both Niels Bohr and Ernest Rutherford in 1913. This post will examine about what is Neil Bohr's Atomic Model, how it not quite the same as Rutherford's Atomic Model, its three proposes and limits.
What is Neil Bohr's Atomic Model?
As indicated by Niels Bohr, his Atomic Model clarified the presence of an Atom which comprises of a little, thick Nucleus which is encircled by circling electrons similar as the construction of the Solar System, where the fascination between the particles is because of electrostatic power as opposed to gravity.
Postulates of Bohr Atomic Model
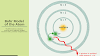
1.Electrons rotate around the nucleus in a fixed roundabout way named "orbit" or "shells" or "energy level." These are the fixed shells. Staying in these levels, electrons don't emit energy.
2.Every roundabout circle will have a specific measure of fixed energy and these round circles were named orbital shells. The electrons won't transmit energy as long as they keep on rotating around the nucleus in the fixed orbital shells.
3.The diverse energy levels are meant by whole numbers, for example, n=1 or n=2 or n=3, etc. These are called as principal quantum numbers. The scope of quantum number may fluctuate and start from the most minimal energy level (core side n=1) to most noteworthy energy level. Get familiar with the idea of an Atomic number here.
4.The diverse energy levels or circles are addressed in two different ways like 1, 2, 3, 4… or K, L, M, N… .. shells. The least energy level of the electron is known as the ground state. Become familiar with the idea of Valency.
5.The change in energy happens when the electrons bounce from one energy level to other. In an iota, the electrons move from lower to higher energy level by obtaining the necessary energy. In any case, when an electron loses energy it moves from higher to bring down energy level.
Consequently,
• 1st circle (energy level) is addressed as K shell and it can hold up to 2 electrons.
• 2nd circle (energy level) is addressed as L shell and it can hold up to 8 electrons.
• 3rd circle (energy level) is addressed as M shell and it can contain up to 18 electrons.
• 4th circle (energy level) is addressed as N Shell and it can contain most extreme 32 electrons.
The circles keep on expanding along these lines.
It is to be noted that the Bohr's atomic model is based on the Quantum Mechanics. About which Niels Bohr said, “Anyone who is not shocked by quantum theory has not understood it.” He also said, “We must be clear that when it comes to atoms, language can only be used as in poetry.”
Limitations of Bohr's atomic model:
1. Bohr regarded electrons as particles where as per de Broglie's theory, having a low mass, electron additionally shows wave nature.
2. Bohr's model was satisfactory just for core having just a single electron for example Hydrogen, He+1, Li+2 and so forth Bohr's model couldn't clarify the spectra of multi-electronic iotas.
3. Bohr's model was two-dimensional where a particle is three-dimensional.
4. Utilizing a superior spectrometer, the spectra showed barely recognizable differences. Bohr's model couldn't clarify the cause of those barely recognizable differences. (Settled by Arthur Sommerfield who envisioned electrons circling in various planes and having curved circles.)
5. Bohr's model couldn't clarify the impact electric field and attractive field on spectra. (Distinct impact and Zeeman impact)
6. In Bohr's condition, the energy and position of electron, rotating around the core were very much characterized. Be that as it may, agreeing, Heisenberg's Uncertainty standard, it is difficult to quantify the position and force of electrons accurately. In the event that the position is measure with greatest accuracy, there will be vulnerability in the estimation of energy and the other way around.




0 Comments