Rutherford's atomic model: experiment, postulates, limitations & examples
Rutherford's Atomic Model Experiment:
In Rutherford's analysis, he barraged high energy floods of α-particles on a meager gold foil of 100 nm thickness. The surges of α-particles were coordinated from a radioactive source. He directed the examination to consider the diversion created in the direction of α-particles after cooperation with the meager sheet of gold. To consider the redirection, he put a screen comprised of zinc sulfide around the gold foil. The perceptions made by Rutherford repudiated the plum pudding model given by J.J. Thomson.
Observation of Rutherford's Gold Foil Experiment:
Based on the observations made during the analysis, Rutherford inferred that:
1.Major space in an atom is vacant – A huge part of α-particles went through the gold sheet without getting redirected. Accordingly, the significant piece of a particle should be vacant.
2.The positive charge in an atom isn't dispersed consistently and it is packed in a very short volume – Few α-particles when assaulted were deflected by the gold sheet. They were deflected minutely and at little points.
3.Not so many α-particles had diverted everywhere points or redirected back. Also, not so many particles had redirected at a straight line. Therefore, he reasoned that the positively charged particles covered a little volume in contrast with the absolute volume of an atom.
Click here to get freelance services.
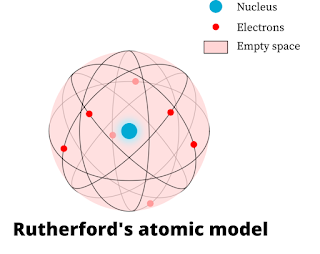
Postulates of Rutherford's atomic model:
1.An atom made out of positively charged particles. Greater part of the mass of an atom was concentrated in a very little area. This area of the atom was called as the core of an atom or the nucleus. It was discovered later that the exceptionally little and thick nucleus consists the positively charged protons and neutrally charged neutrons.
2.The nucleus of an atom is encircled by negatively charged particles called electrons. The electrons spin around the nucleus in a fixed round way at high velocity. These fixed roundabout ways were named as "orbits".
3.An atom has no net charge or they are electrically nonpartisan since electrons are contrarily charged and the thickly focused core is decidedly charged. A solid electrostatic power of attractions holds together the nucleus and electrons.
4.The size of the nucleus of an atom is very little in contrast with the complete size of an atom.
Limitations of the Rutherford's atomic model:
1.Rutherford's model couldn't clarify the strength of a particle. As indicated by Rutherford's propose, electrons rotate at a fast around the nucleus of an atom in a fixed circle. In any case, Maxwell clarified quickened charged particles discharge electromagnetic radiations. Subsequently, electrons spinning around the nucleus will deliver electromagnetic radiation and fall into the nucleus. Thus, the atom will lose it's existence which doesn't happen in reality.
2.The electromagnetic radiation will have energy from the electronic movement because of which the circles will progressively recoil. At long last, the circle will break down into the nucleus. As per the estimations, if Maxwell's clarification is followed Rutherford's model will implode with 10-8 seconds. Accordingly, Rutherford nuclear model was not after Maxwell's hypothesis and it couldn't clarify an atom's existence.
3.Rutherford's hypothesis was fragmented on the grounds that it didn't make reference to anything about the course of action of electrons in the circle. This was one of the significant downsides of Rutherford nuclear model.
4. The model was based on the general mechanics.
5. The model couldn't describe the spectrum in general.
To fix these problems, Bohr proposed another model which was based on the Quantum Mechanics.




0 Comments