Einstein's explanation of photoelectric effect
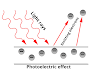
The photoelectric effect is a wonder where electrons are radiated from the metal surface when the light of adequate recurrence is episode upon. The idea of the photoelectric effect was first recorded in 1887 by Heinrich Hertz and later by Lenard in 1902. Yet, both the perceptions of the photoelectric effect couldn't be clarified by Maxwell's electromagnetic wave hypothesis of light. Hertz (who had demonstrated the wave hypothesis) himself didn't seek after the matter as he felt sure that it very well may be clarified by the wave hypothesis. Be that as it may, the idea fizzled in the accompanying records:
Click here to get premium services.
1.As per the wave hypothesis, energy is consistently disseminated across the wavefront and is reliant just on the force of the pillar. This infers that the dynamic energy of electrons increments with light force. In any case, the dynamic energy was autonomous of light power.
Click here to read Bohr's atomic model.
2.Wave hypothesis says that light of any recurrence ought to be equipped for shooting electrons. In any case, electron discharge happened distinctly for frequencies bigger than a limit recurrence.
3.Since energy is reliant on force as per wave hypothesis, the low-power light ought to discharge electrons after some time with the goal that the electrons can procure adequate energy to get radiated. In any case, electron emanation was unconstrained regardless of how little the force of light.
Einstein settled this issue utilizing Planck's progressive thought that light was a molecule. The energy conveyed by every molecule of light (called quanta or photon) is subject to the light's recurrence (ν) as appeared:
E = hν
Where h = Planck's consistent = 6.6261 × 10-34 Js.
Since light is packaged up into photons, Einstein conjectured that when a photon falls on the outside of a metal, the whole photon's energy is moved to the electron.
A piece of this energy is utilized to eliminate the electron from the metal molecule's grip and the rest is given to the catapulted electron as motor energy. Electrons discharged from under the metal surface lose some motor energy during the crash. However, the surface electrons convey all the dynamic energy bestowed by the photon and have the most extreme active energy.
We can compose this numerically as:
Energy of photon
= energy needed to launch an electron (work) + Maximum active energy of the electron
E = W + KE
hv = W + KE
KE = hv – w
At the limit recurrence, ν0 electrons are simply launched out and don't have any motor energy. Underneath this recurrence, there is no electron discharge. Hence, the energy of a photon with this recurrence should be the work capacity of the metal.
w = hv0
Accordingly, Maximum dynamic energy condition becomes:
KE = 1/2mv2max=hv–hv0
1/2mv2max=h(v−v0)
Vmax is the most extreme dynamic energy of the electron. It is determined tentatively utilizing the halting potential. If it's not too much trouble, read our article on Lenard's perceptions to comprehend this part.
Halting potential = ev0 = 1/2mv2max
Accordingly, Einstein clarified the Photoelectric impact by utilizing the molecule idea of light.




0 Comments